When considering materials for heat transfer applications, both heat conductivity aluminum and copper often come up. These metals are widely known for their ability to conduct heat, making them essential in various industries. However, there are some key differences between the two. While copper is generally recognized for its superior heat conductivity, aluminum offers distinct advantages that make it a popular choice in specific contexts. In this blog, we’ll explore the heat conductivity of aluminum, compare it with copper, and discuss why aluminum remains a top contender in many applications.
What is the Heat Conductivity of Aluminum?
Heat conductivity refers to a material’s ability to conduct heat. Aluminum, while not as conductive as copper, still performs remarkably well in transferring heat. The thermal conductivity of aluminum is about 205 W/m·K, which, although lower than copper’s 385 W/m·K, is still sufficient for many applications. This makes aluminum a practical choice for applications where efficient heat dissipation is required.
Aluminum’s thermal conductivity varies slightly depending on the alloy. For example, 6061 aluminum, a commonly used alloy, has a thermal conductivity of about 167 W/m·K. Despite this, aluminum remains a reliable material for heat sinks, radiators, and other components requiring effective heat management.
Is Aluminum a High Conductor of Heat?
Aluminum is considered a good conductor of heat, though it’s not the best. Its heat conductivity is higher than many other metals, such as steel or titanium, but lower than copper. This balance of conductivity and other properties, such as weight and corrosion resistance, makes aluminum a versatile material. In applications where weight is a concern, aluminum’s lower density compared to copper is a significant advantage. This is why aluminum is often chosen for automotive and aerospace applications, where weight reduction is critical.
The combination of adequate heat conductivity and lightweight properties makes aluminum an excellent choice for heat exchangers, power electronics, and other components where both heat dissipation and weight are critical factors.

What is the Thermal Conductivity of Aluminum in K?
The thermal conductivity of aluminum is usually expressed in watts per meter-kelvin (W/m·K). For pure aluminum, the thermal conductivity is approximately 205 W/m·K at room temperature. This value can vary depending on the specific alloy and its temperature. For instance, as the temperature increases, the thermal conductivity of aluminum decreases slightly, but it remains effective for most applications.
Aluminum’s ability to maintain a relatively stable thermal conductivity across different temperatures is one reason it’s preferred in environments where temperature fluctuations are common. Whether in electronics or construction, aluminum’s consistent performance makes it a reliable material for heat management.
What is the Thermal Conductivity of 6061 Aluminum?
6061 aluminum is one of the most widely used aluminum alloys due to its excellent mechanical properties and versatility. Its thermal conductivity is slightly lower than that of pure aluminum, typically around 167 W/m·K. This alloy is often used in applications where a combination of good thermal conductivity, strength, and corrosion resistance is needed.
Despite having lower thermal conductivity than pure aluminum, 6061 aluminum is still effective in dissipating heat. Its ease of machining and ability to be heat-treated makes it a popular choice in the automotive and aerospace industries, where it’s used in components like engine parts and structural frames.

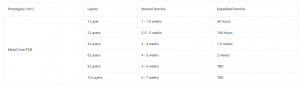
Basic Parameters of 6061 Series Aluminum
| Property | Parameter | 6061 Aluminum |
| Chemical Composition | Aluminum (Al) | 95.85% – 98.56% |
| Magnesium (Mg) | 0.8% – 1.2% | |
| Silicon (Si) | 0.4% – 0.8% | |
| Copper (Cu) | 0.15% – 0.4% | |
| Chromium (Cr) | 0.04% – 0.35% | |
| Iron (Fe) | ≤ 0.7% | |
| Zinc (Zn) | ≤ 0.25% | |
| Manganese (Mn) | ≤ 0.15% | |
| Titanium (Ti) | ≤ 0.15% | |
| Other Elements | Each ≤ 0.05%, Total ≤ 0.15% | |
| Mechanical Properties | Ultimate Tensile Strength (UTS) | 290 MPa (42,000 psi) |
| Yield Strength (0.2% offset) | 241 MPa (35,000 psi) | |
| Elongation | 8% – 12% (depending on temper) | |
| Hardness (Brinell) | 95 HB (T6 condition) | |
| Shear Strength | 207 MPa (30,000 psi) | |
| Physical Properties | Density | 2.70 g/cm³ (0.0975 lb/in³) |
| Melting Point | 582°C – 652°C (1,080°F – 1,206°F) | |
| Thermal Conductivity | 167 W/m·K | |
| Coefficient of Thermal Expansion | 23.6 µm/m·°C (13.1 µin/in·°F) | |
| Electrical Conductivity | 40% IACS | |
| Specific Heat | 0.896 J/g·°C |
Why Do Conductors Have a High Heat Capacity?
Conductors typically have a high heat capacity because of their ability to absorb and distribute heat energy efficiently. This property is particularly important in materials used for heat dissipation. While aluminum has a lower heat capacity compared to copper, it still performs well enough to be used in many thermal management applications.
The high heat capacity of aluminum allows it to absorb heat without a significant rise in temperature, making it effective in applications like heat sinks and radiators. Its ability to distribute heat evenly across its surface ensures that hot spots are minimized, contributing to the overall efficiency of the cooling process.
Which Metal is the Best Conductor of Heat?
Copper is widely recognized as the best conductor of heat among common metals, with a thermal conductivity of about 389 W/m·K. This makes it ideal for applications where maximum heat transfer is required. However, copper is heavier and more expensive than aluminum, which can be a disadvantage in certain contexts.
Aluminum, while not as conductive as copper, offers a balance of good heat conductivity, low weight, and cost-effectiveness. In applications where these factors are more important than the absolute best conductivity, aluminum is often the preferred choice. This is why aluminum is commonly used in heat sinks, air conditioning units, and automotive radiators, where its combination of properties provides the best overall performance.
Choosing between aluminum and copper depends on the specific requirements of the application. If weight and cost are significant considerations, aluminum often comes out on top. However, for situations where the highest possible heat transfer is needed, copper might be the better option.